Introduction: Patient-reported outcomes (PRO) provide meaningful insight into patient perspectives on treatment effect. Clinically relevant outcomes such as improvements in overall survival (OS) and quality of life (QOL) should guide clinical decision-making, furthermore, the FDA encourages the implementation of patient-centric PRO measures in clinical trials.
Objectives: To evaluate the frequency at which PRO measures included in clinical trials, are made publicly available when trial results are published.
Methods: We searched Citeline® Trialtrove database, a registry of clinical trials, for randomized phase 2/3 and 3 clinical trials evaluating patients with hematological malignancies, completing enrollment between the years 2007-2017, for which PRO endpoints were listed. We excluded trials evaluating supportive care. We recorded the following data: indication, treatment and comparator, phase, primary endpoint, type of scale or questionnaire used for PRO endpoint. We then identified all available publications associated with the trial, and recorded type of publication (abstract or full text), year, reported outcomes, and 13 criteria of CONSORT-PRO that reflect the completeness of reporting.
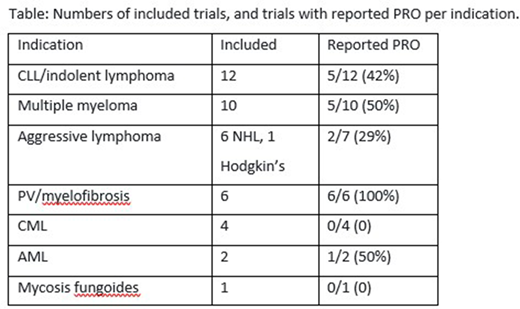
Results: We identified 362 trials through our search. 53 trials were listed as including at least one PRO endpoint, of which 8 were never published. Indications are listed in Table. PRO endpoints were assessed utilizing 26 PRO different tools, 7 were disease specific, 2 were treatment specific. PRO was the primary outcome in two trials. EORTC-QLQ-30 was most frequently used tool. Study sponsor was industry-only in 21 trials, industry-academic in six, and academic in 18. Of the 45 trials analyzed, only 19 (42%) published any PRO data. Eleven of all trials (24%) were judged as comprehensively reporting PRO. The median number of CONSORT-PRO quality indicators was eight criteria. Of the 45 trials, 12 provided information about missing PRO data. Of the 15 trials that showed PFS benefit with no OS benefit, 8(53%) did not publish any results the PRO, to support a patient centric outcome. 36 of the trials were published as full-text, and nine as abstract. In considering the 36 full text publications, 17 (47%) did not report any PRO. Nine of the full-text publications, reported PRO as part of the primary publication or within the following 6 months.
Conclusions: Despite a growing emphasis on QOL and use of PROs in oncology clinical trials, and despite patient and health provider efforts to record PRO data, most hematologic malignancies randomized trials still do not report the PRO endpoints. In several cases they were published later and in a partial manner, minimizing their impact on treatment decisions. This may indicate a disregard to PRO data collected, incomplete collection or methodological flaws in data analysis. Regardless of the reason, PRO data should be routinely included in study publications to allow for a complete assessment of investigational treatment outcome - including disease related outcomes, as well as those reported by the patients themselves.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.